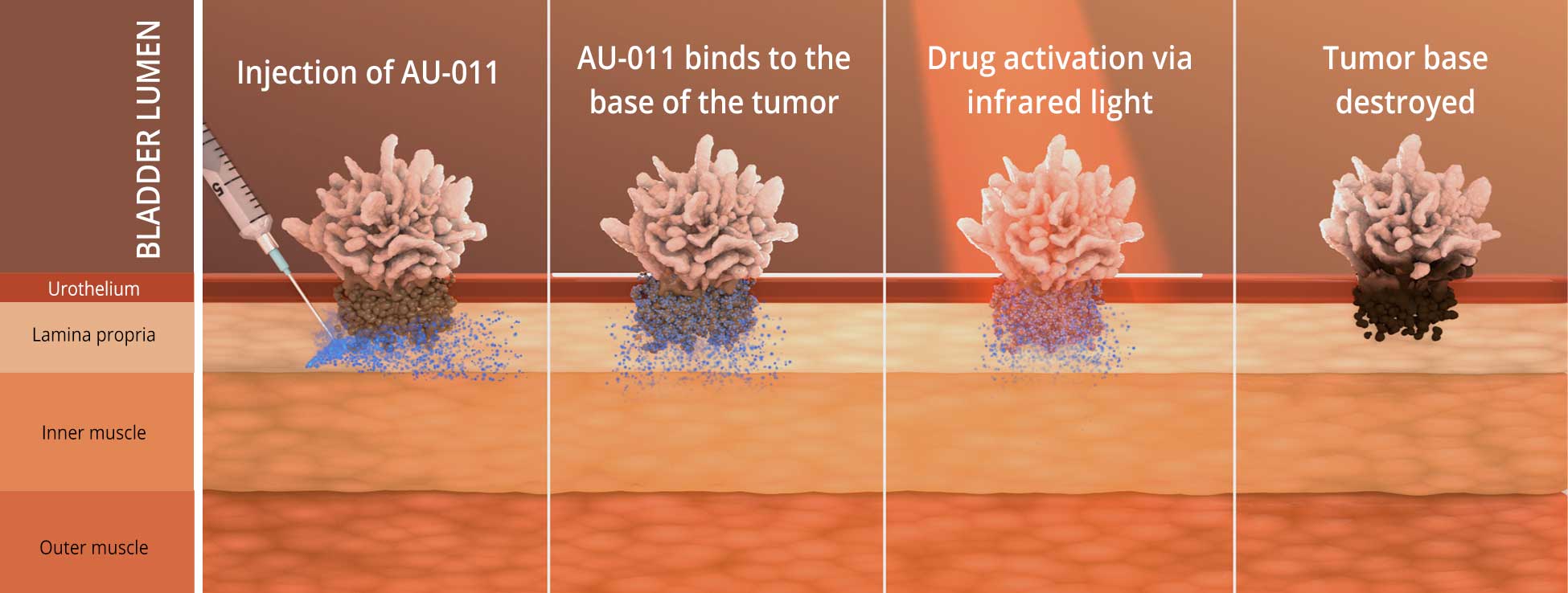
NON-MUSCLE INVASIVE AND MUSCLE INVASIVE BLADDER CANCER
Pioneering a novel approach to treat a high unmet medical need.
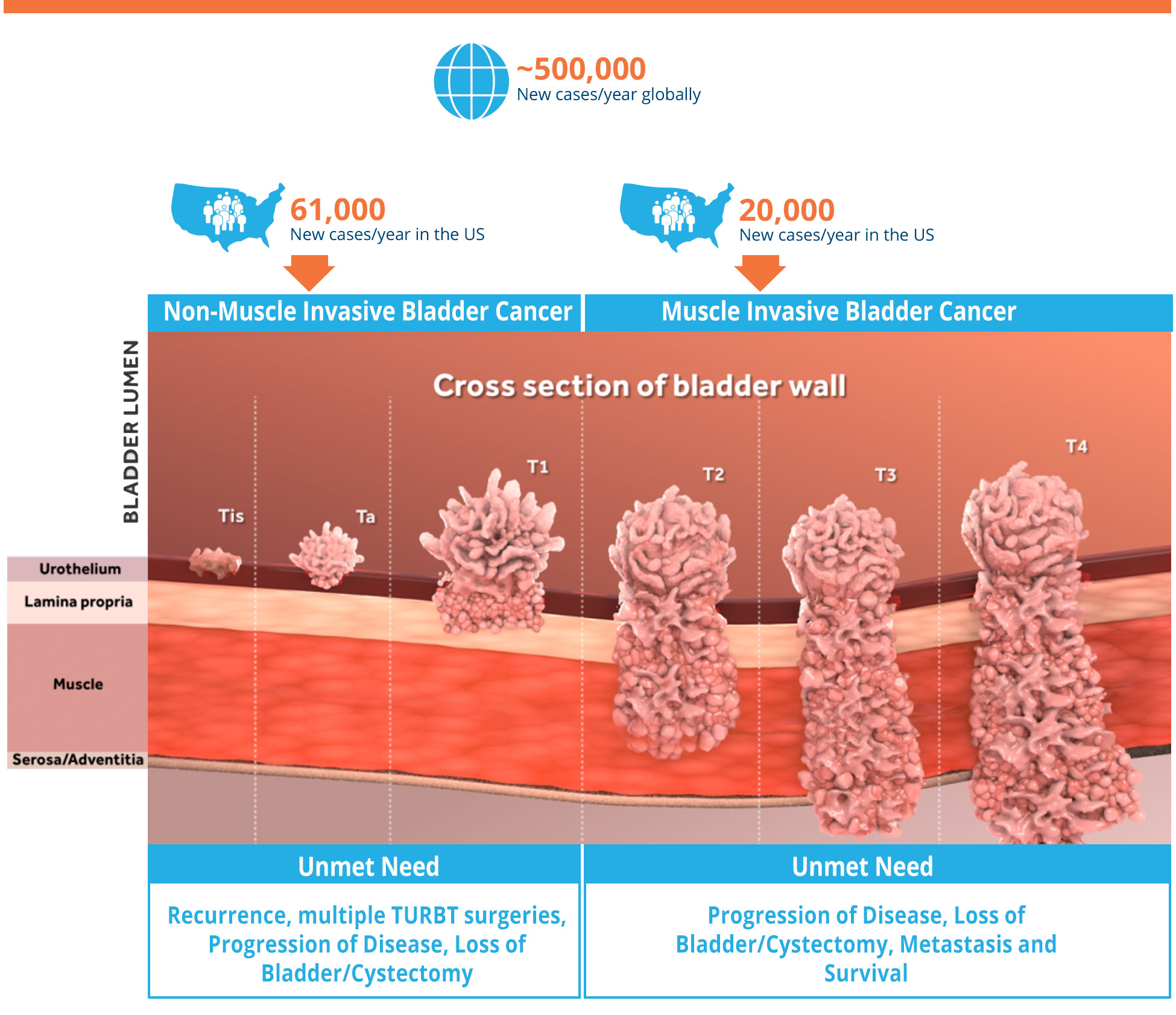
Bladder cancer is the most common malignancy involving the urinary system. Non-muscle invasive bladder cancer, or NMIBC and muscle invasive bladder cancer, or MIBC has a yearly incidence of 81,000 patients in the US. NMIBC occurs in the tissue that lines the inner surface of the bladder and where the bladder muscle is not yet involved. NMIBC and MIBC are currently treated with a combination of surgery, immunotherapy, and chemotherapy, and unfortunately a large proportion of subjects may progress to needing cystectomy, or removal of the bladder.