Patients must choose between their life and their vision.
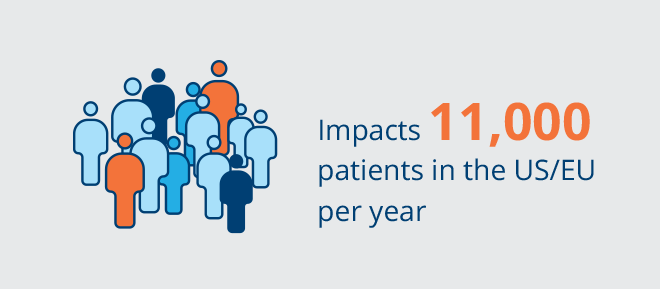
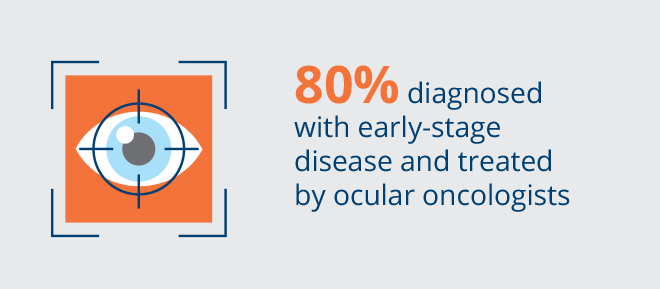
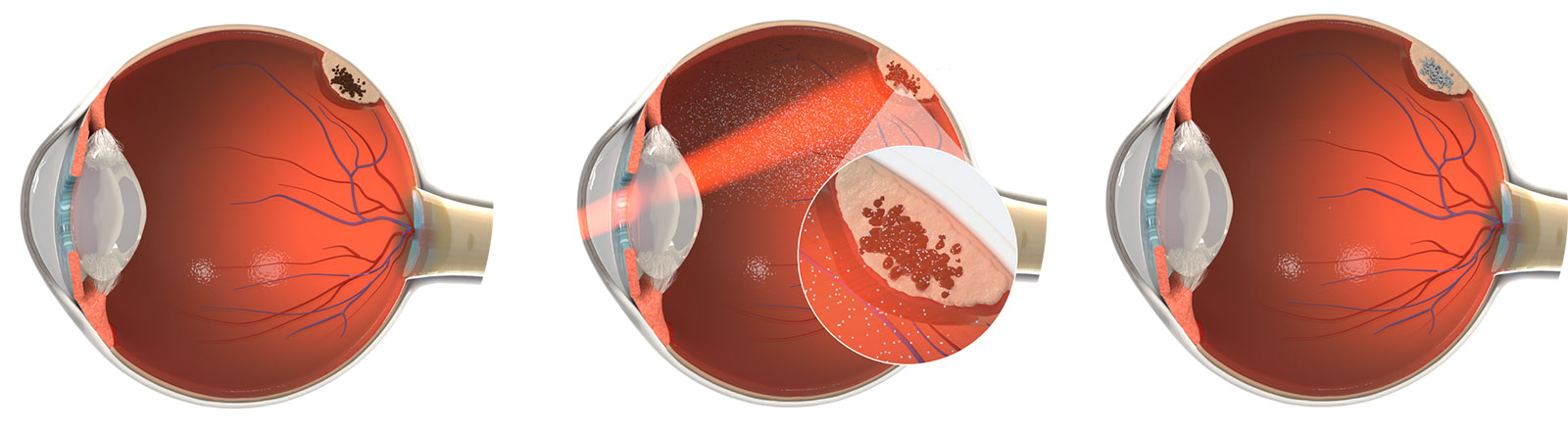
Choroidal melanoma (CM) is the most common intraocular cancer in adults, with an incidence of approximately 11,000 patients per year in the United States and Europe. CM comprises approximately 90% of all cases of uveal melanoma, consisting of melanomas in the choroid, ciliary body and iris, which are collectively referred to as the uvea. It is estimated that 96% of patients are diagnosed early without clinical evidence of metastatic disease. There are approximately 2,000 new cases of choroidal melanoma treated each year in the United States and 1,600 new cases treated each year in Europe. However, there is a high number of patients with indeterminate lesions that are considered an early-stage diagnosis of melanoma that are not treated immediately given the lack of vision preserving therapies. Despite the current treatments with radiotherapy, the long-term prognosis is poor with death occurring in more than 50% of cases. There are no FDA-approved therapies for choroidal melanoma. We intend to develop belzupacap sarotalocan (bel-sar), our first virus-like drug conjugate (VDC), as a first line therapy to treat early-stage disease, which includes small melanomas and indeterminate lesions representing approximately 9,000 patients in the United States and Europe.