A NOVEL TARGETED ONCOLOGY PLATFORM BASED ON VIRUS-LIKE PARTICLES
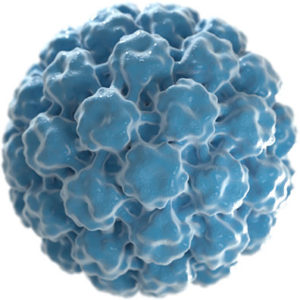 Our technology platform represents a novel approach to target a broad range of solid tumors using virus-like particles, or VLPs, derived from the human papillomavirus (HPV) that are designed to enable their use as oncology therapeutics. Our VLPs can be loaded or conjugated with drugs creating a new class of targeted therapies.
Our technology platform represents a novel approach to target a broad range of solid tumors using virus-like particles, or VLPs, derived from the human papillomavirus (HPV) that are designed to enable their use as oncology therapeutics. Our VLPs can be loaded or conjugated with drugs creating a new class of targeted therapies.